Label Each Carbon Atom With the Appropriate Hybridization.
The 2s and the three 2p orbitals hybridise together and each orbital will be completed by adding one more electron from sharing with N H H and the other C. The geometry of hybridized carbon atom is trigonal planar.
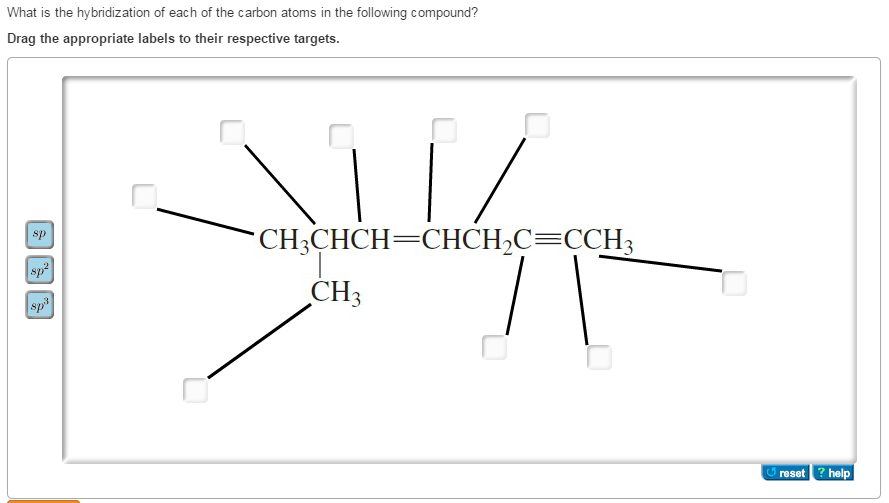
Solved What Is The Hybridization Of Each Of The Carbon Atoms Chegg Com
Show transcribed image text Expert Answer.
. Comments 0 Answer. Of 20 Label each carbon atom with the appropriate hybridization. The geometry of sp hybridized carbon atom is linear.
It is sp3 and sp2 This is because the first carbon has formed four bonds. For the compound the electronic configuration of the atoms carbon and hydrogen are. Predict the molecular shape of methane the carbonate ion carbon dioxide and the sulfite ion cover left side of screen.
Label each carbon atom with the appropriate hybridization cover bottom. Mixing of pure orbitals having nearly equal energy to form equal number of completely new orbitals is said to be hybridization. All single bonds are bonds.
Can you label each carbon atom with the appropriate hybridization. An isolated sp hybridized carbon atom for viewing. This molecule has a total of 3 bonds.
Label each carbon atom with the appropriate hybridization. The hybrid orbitals of carbon involve in bond formation with hydrogen. The central atom is surrounded by two electron groups and is involved in two bonds so it is sp hybridized.
Get more out of your subscription. 2pz 2px Carbon. Spsp2 or sp3 label each carbon atom with the appropriate hybridization.
In this video we use both of these methods to determine the hybridizations of atoms in various organic molecules. So as you can see from the picture one electron from 2s orbital moves to the empty 2pz orbital. While the second carbon is sp2.
See the answer See the answer See the answer done loading. The geometry of hybridized carbon atom is tetrahedral. If you cant find your institution please check your spelling and do not use abbreviations.
The carbon-hydrogen bonds in cyclohexane are always eclipsed. Can you label each carbon atom with the appropriate hybridization. All the bonds in the compound is single.
Sp2 sp2 sp3 sp sp sp3. Therefore each carbon atom is sp3 hybridized. We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number.
Hence the geometry at each carbon depends on the type of hybridization. Who are the experts. This problem has been solved.
All of the carbon atoms are in s p 3 hybridization forming a hexagonal network and the hydrogen atoms are bonded to carbon on both sides of the plane in an alternating manner. The geometry of hybridized carbon atom is linear. The geometry of sp2 the hybridized carbon atom is trigonal planar.
Starting on the left the hybridizations are. However when forming sigma bonds the carbon atoms combine the four atomic orbitals into four molecular orbitals. The electrons of each carbon atom are found in one s-orbital and three p-orbitals.
Label each carbon atom with the appropriate hybridization. A bonded carbon atom would need orbital overlap for each orbital present spa spb 2pz and 2px. Up to 256 cash back Get the detailed answer.
This results in each carbon now having four hydridized sp3 orbitals. Answer to label each carbon atom with the appropriate hybridization. Label each carbon atom with the appropriate hybridization.
Label each carbon atom with the appropriate hybridizationspsp2 or sp3 Label each carbon. Label each carbon atom with the appropriate hybridization. The hybrid orbitals of carbon involve in bond formation with hydrogen.
There remain two 2p orbitals which are perpendicular to the two sp hybrid orbitals and to each other. Chemistry Science Organic chemistry. Double bonds have 1 bond and triple bonds have 2 bonds.
Label each carbon atom with its optimum c c c bond angle. Hence the geometry at each carbon depends on the type of hybridization. Sp hybridized CH3 Answer Bank sp hybridized sp hybridized ECH sp hybridized sp hybridized at sp hybridized CH sp hybridized lo Tv sp hybridized ČH3 an wit pai PrtScn Home End F10.
The geometry of sp3 hybridized carbon atom is tetrahedral. Each 2p orbital extends along its entire axis with opposite phase in each lobe. This is the currently selected item.
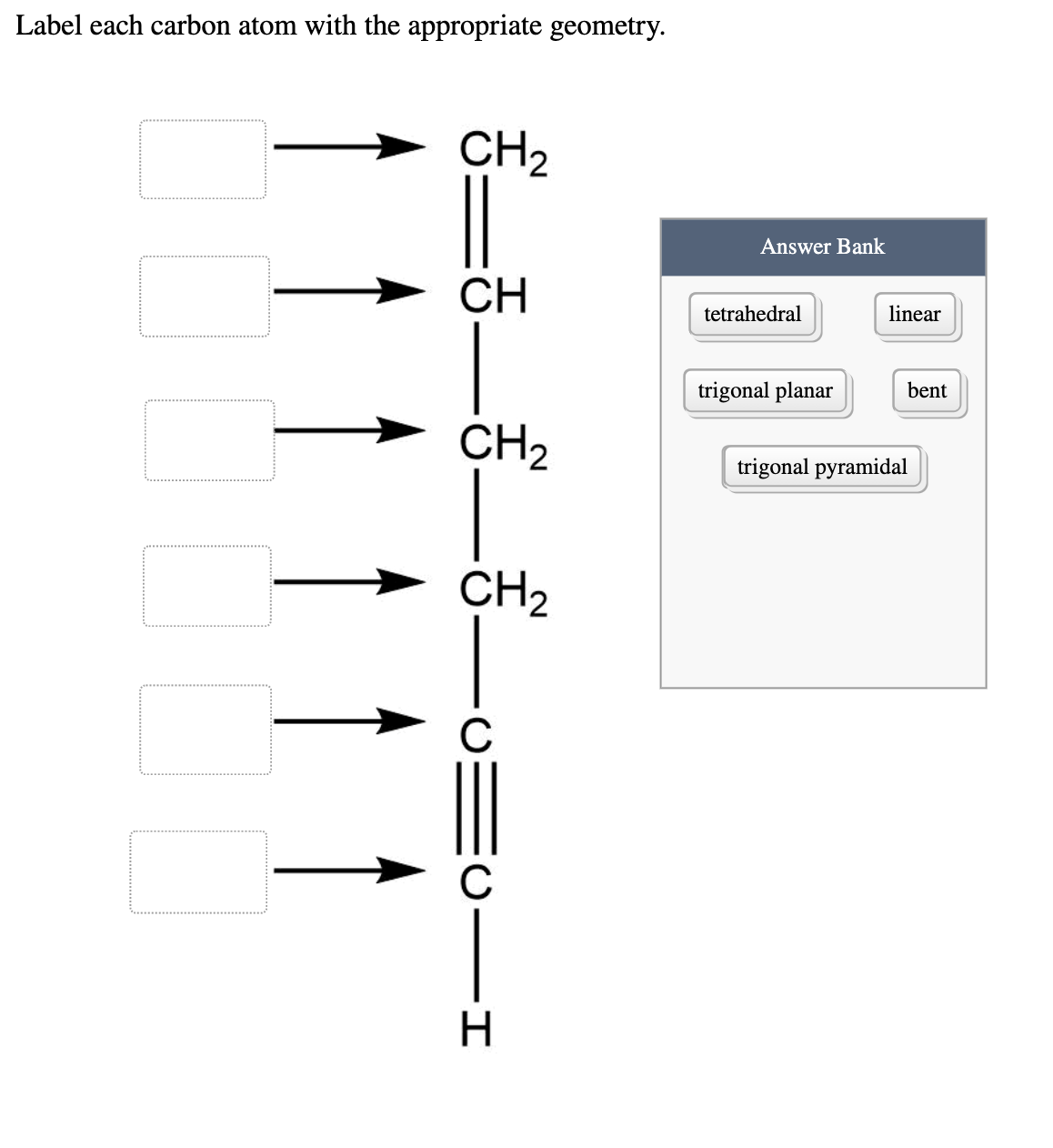
This molecule has 8 C-H bonds and 5 C-C bonds for a total of 13 bonds. Label each carbon atom wiht the appropriate geometry cover right side Sapling Hw Ch 121. Double and triple bonds each contain 1 bond.
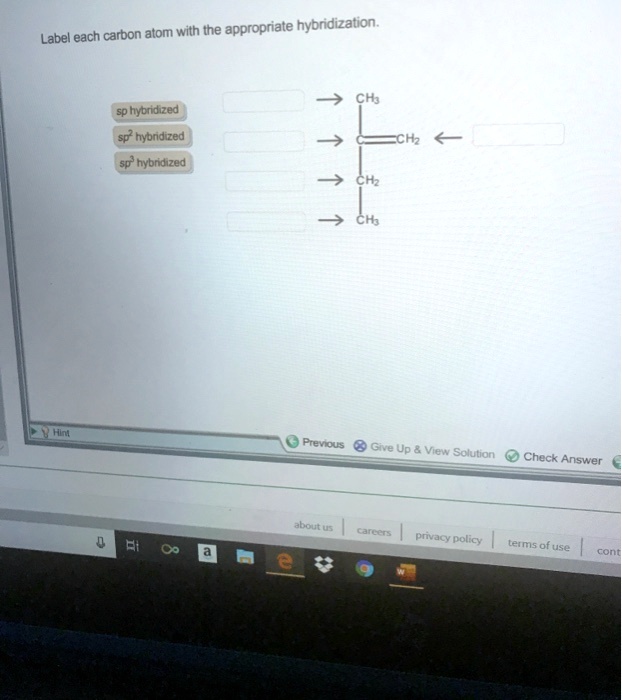
Answered Label Each Carbon Atom With The Bartleby
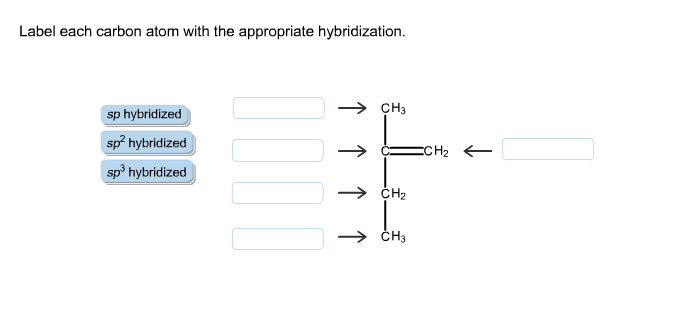
Solved Label Each Carbon Atom With The Appropriate Chegg Com
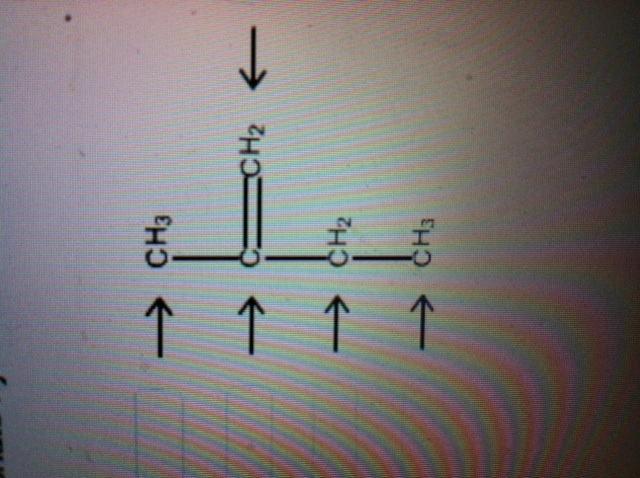
Solved Label Each Carbon Atom With The Appropriate Chegg Com

Hybrid Atomic Orbitals Chemistry I
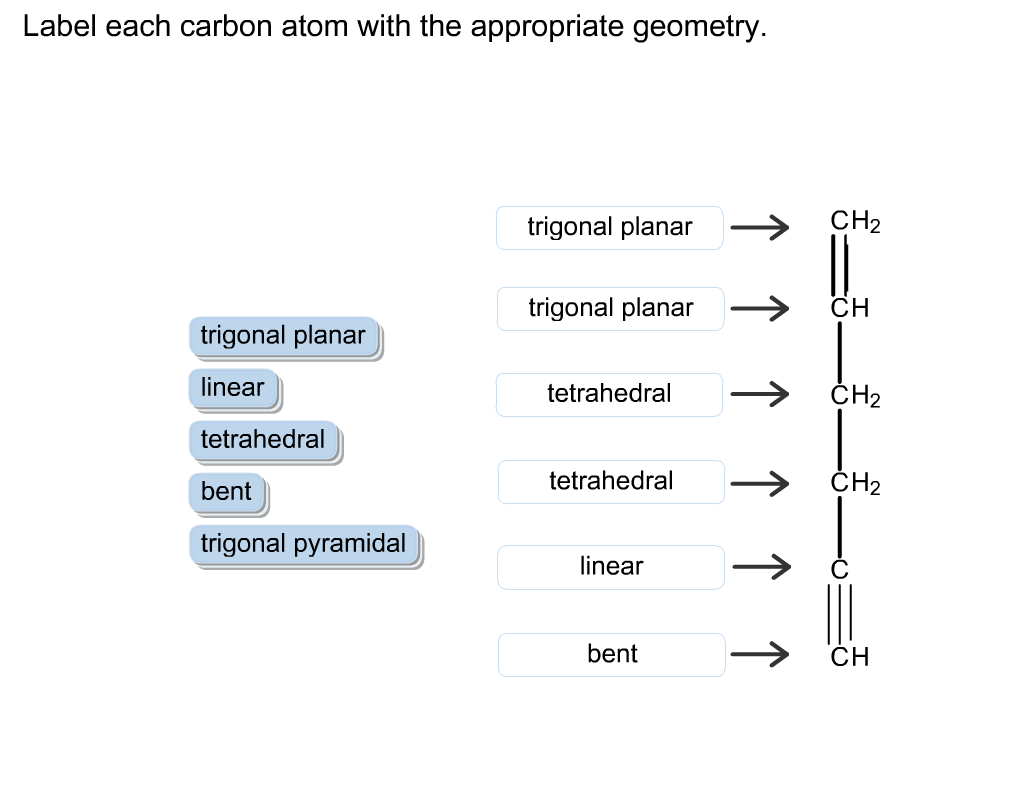
Solved Label Each Carbon Atom With The Appropriate Geometry Chegg Com
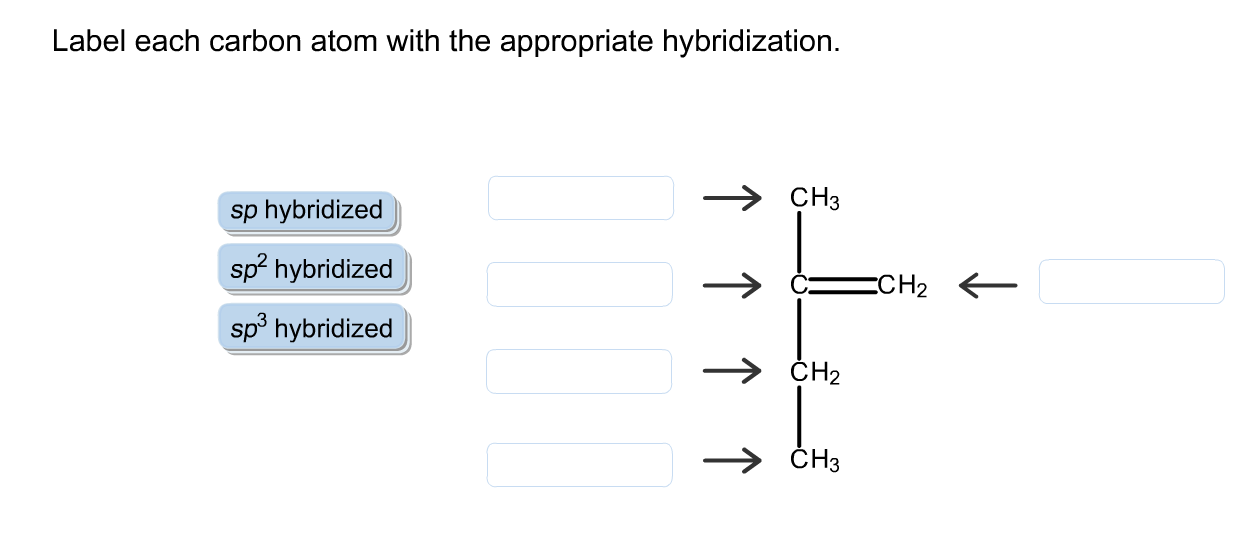
Solved Label Each Carbon Atom With The Appropriate Chegg Com
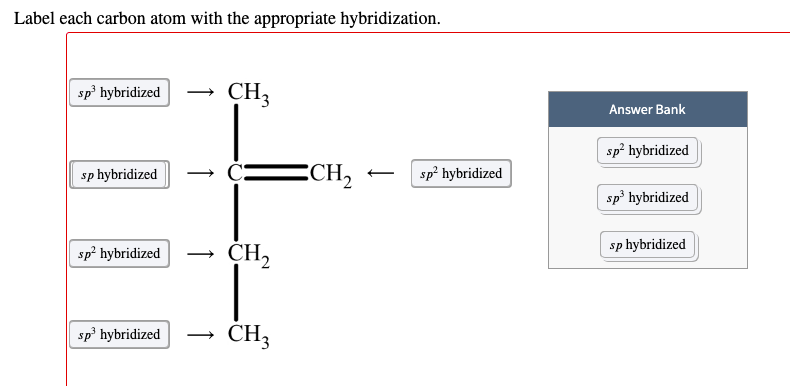
Answered Sp Hybridized Ch3 Sp Hybridized Ech Bartleby
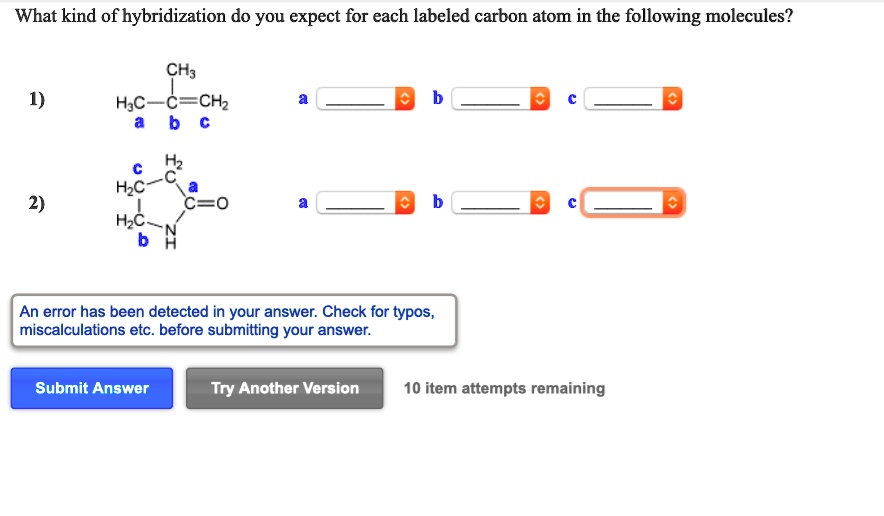
Solved What Kind Of Hybridization Do You Expect For Each Labeled Carbon Atom In The Following Molecules Hjc C Chz Hz Hzc C 0 Hzc B N An Error Has Been Detected In
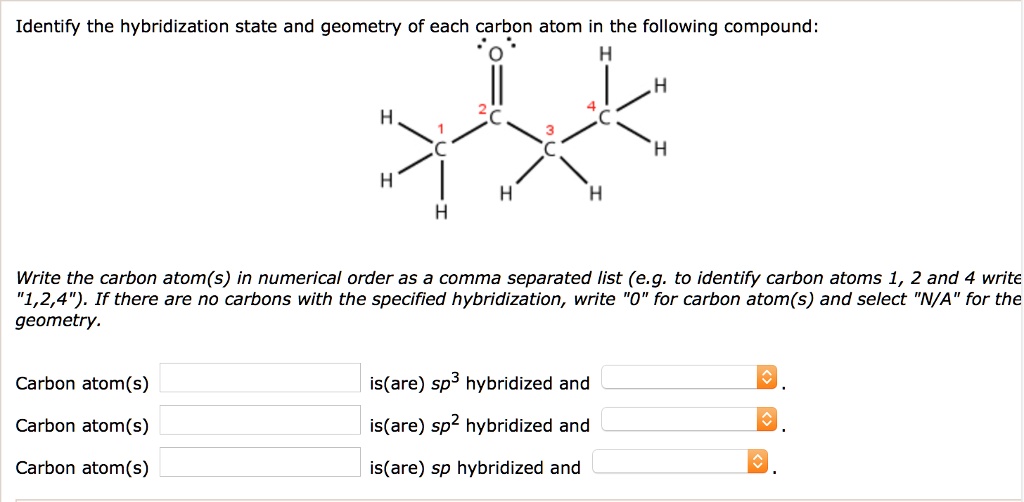
Solved Identify The Hybridization State And Geometry Of Each Carbon Atom In The Following Compound Write The Carbon Atom S In Numerical Order As A Comma Separated List E G To Identify Carbon Atoms 1

Label Each Carbon Atom With The Appropriate Geometry Label Each Carbon Atom With The Appropriate Geometry Homeworklib
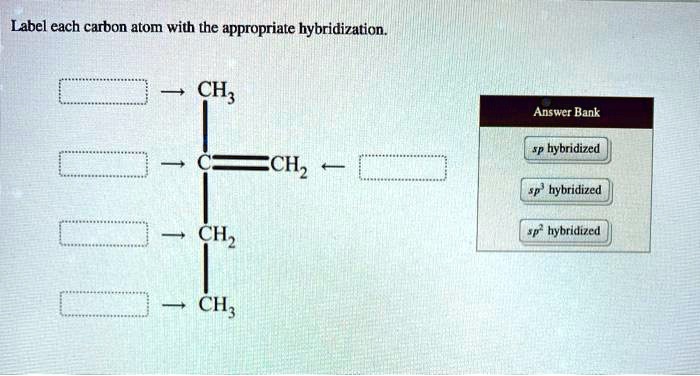
Solved Label Each Carbon Atom With The Appropriate Hybridization Ch Answer Bank Sp Hybridized Chz Hybridized Ch Hyhridved Ch
Solved Label Each Carbon Atom With The Appropriategeometry Course Hero
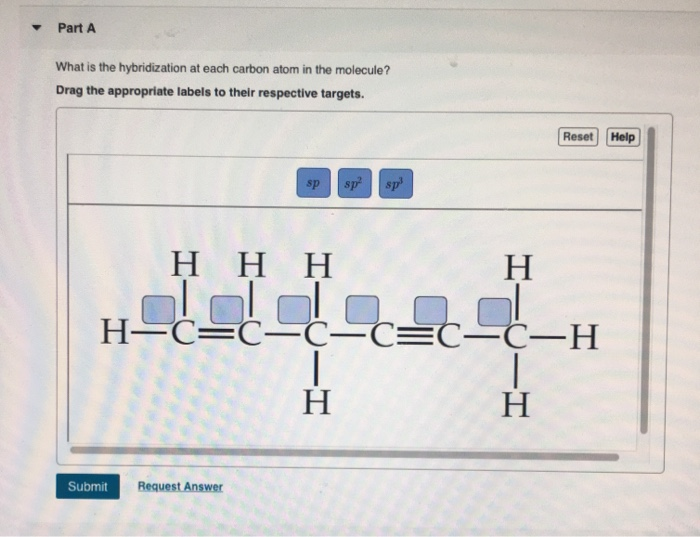
Solved Part A What Is The Hybridization At Each Carbon Atom Chegg Com

Ochem Spring 2017 Exam 1 Flashcards Quizlet
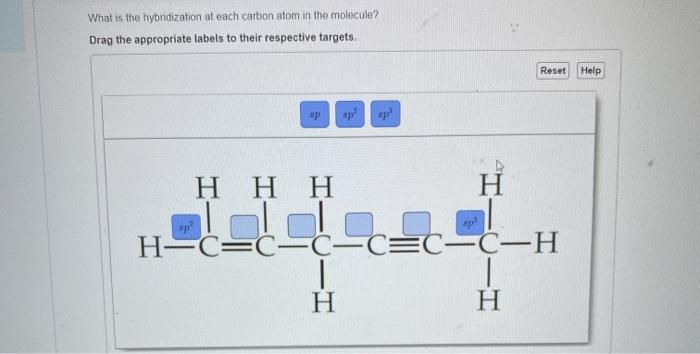
Solved What Is The Hybridization At Each Carbon Atom In The Chegg Com
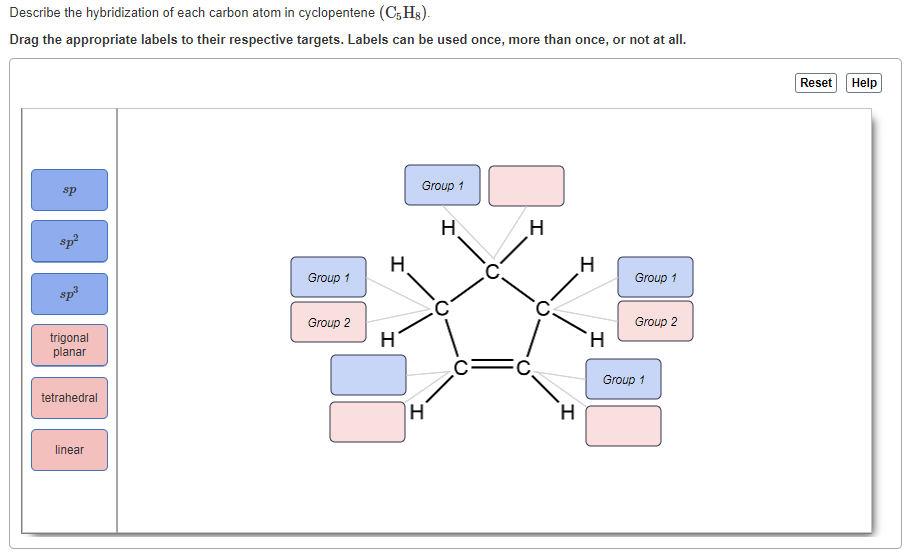
Solved Describe The Hybridization Of Each Carbon Atom In Chegg Com

Solved Each Carbon Atom With The Appropriate Hybridization Label Chj Hybridized Hybndized Chz Hybrdized Chz Chj Previous 8 Give Up View Solution Check Answer Aboutue A Pmaicy Policy Tetms Of Use
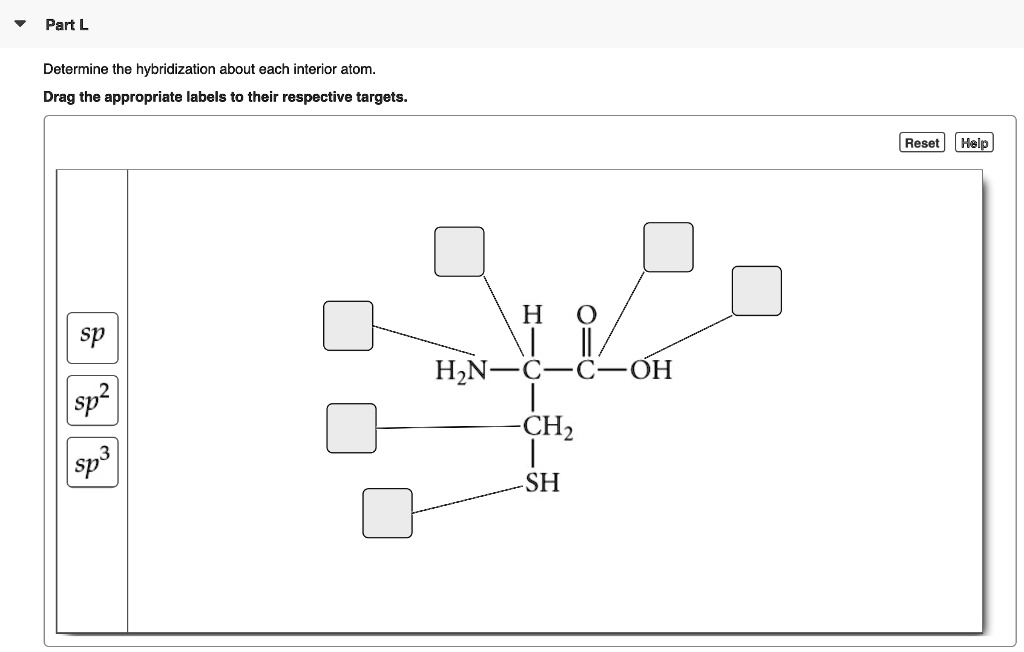
Solved Part L Determine The Hybridization About Each Interior Atom Drag The Appropriate Labels To Their Respective Targets Reset Help Sp Hzn Oh Sp Chz Sp3 Sh
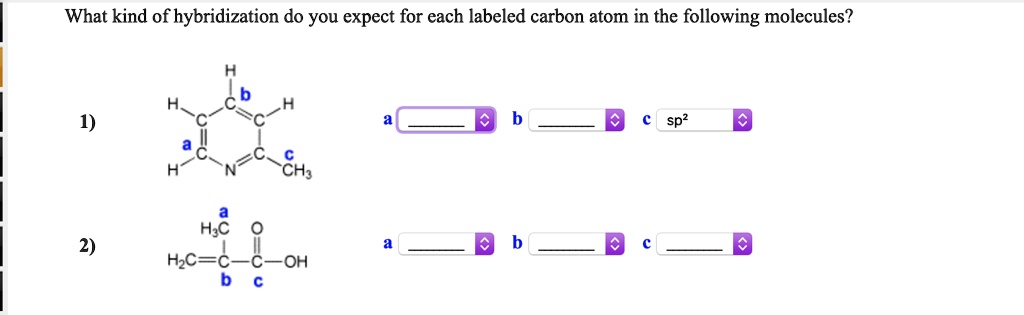
Solved What Kind Of Hybridization Do You Expect For Each Labeled Carbon Atom In The Following Molecules Hic Hc C Oh Ch
Comments
Post a Comment